Not much is known about the infamous Avogadro besides his contributions to Chemistry and Physics. Avogadro was born on August 9th, 1776 as the son of the Count of Quaregna (a title he later inherited). Avogadro spent most of his life in his hometown of Turin, Italy. He later became a professor at the Turin University. Avogadro had six children with a loving wife, and was a strong religious man. He was said to be liked by his students because of his sense of humor also. Avogadro started college when he was only 13, and graduated when he was 16. Avogadro received his doctorates in ecclesiastical law (law pertaining to the church) at the ripe age of 20. Following that, he began to practice law. Although Amedeo Avogadro was destined to become a lawyer, his main interests changed to chemistry, mathematics, and physics.
In the year 1800, Avogadro began his private studies in Physics and Mathematics. In his free time he did a lot of reading and had a complete set of the current scientific journals in his library printed in four

The Avogadro constant or (the Avogadro number earlier) is the number of elementary units in one mole of any substance. The Avogadro constant is denoted as NA. It has the dimension of the reciprocal amount of substance (mol −1). The approximate value of NA is 6.022 × 10 23 mol −1. Amedeo Avogadro was born in Turin to a noble family of the Kingdom of Sardinia (now part of Italy) in the year 1776.He graduated in ecclesiastical law at the late age of 20 and began to practice. Soon after, he dedicated himself to physics and mathematics (then called positive philosophy), and in 1809 started teaching them at a liceo (high school) in Vercelli, where his family lived and had. Amedeo Avogadro. If you were to sit in a chemistry class you wouldn't get very far without using Avogadro's number. Avogadro's number equals 6.02 x 10^23.
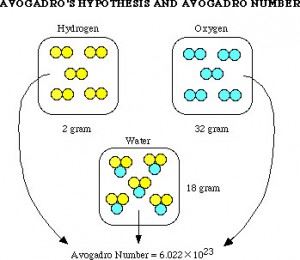
different languages. In one of these journals he read in 1808 that Joseph Louis Gay-Lussac, “Had found that when two gasses react together to form different products, the volumes of the reactants and the products (if they are all still gasses) are all whole numbers.” This accepted belief was called the Law of Combining Volumes. Avogadro then thought, hey, this must mean that equal amounts of gas at the same temperature and pressure must contain the same amount of molecules. He knew that when two volumes of Hydrogen and one volume of oxygen were reacted it formed two volumes of water vapor. Why two and not one? Avogadro’s answer was that it must mean that sets of two oxygen atoms were used to create the one volume of oxygen he used. After noticing this, Avogadro was able to see that, “Molecular weights of any set of gasses are the same as the ratio of the densities to those gasses under the same conditions of temperature and pressure.” His conclusion was that, “All gasses, simple or complex, contain the same number of molecules under the same conditions of temperature and pressure.” This idea is one of the main points in Chemistry. Although Avogadro did not literally find the unit of Moles (6.02 x 10^23 molecules), the number is called Avogadro’s Number in honor of his theories that led future scientists to be able to calculate this number.

Avogadro's Law Formula
The impact of this amazing realization is still spreading today. Avogadro’s law is used in thousands of different applications each and every day; from a high school chemistry I class, to scientists trying to figure out the cure to cancer. This number, and law is used to find the outcome of a reaction before it is even attempted, and is the basis of many theoretical chemistry and physics ideas. Although the applications for this are limited to chemical equations and the such, the combinations are endless, straying into the realm of the chemistry of DNA and other complicated molecular structures. We are only beginning to use this law to its full extent, and we will continue to broaden its impact by using it more.

Amedeo Avogadro's Number
Amedeo Avogadro was a bright man who stumbled upon a question chemists had encountered for years and somehow, with some great brain power, found the answer. The only dilemma with this historic find was that Avogadro’s life was, for the most part, isolated from other scientists. This was one of the main reasons why his theory did not catch on quickly. In fact, it was not until 1858 when the significance of Avogadro’s work was realized. When Italian chemist Stanislao Cannizzaro explained why Avogadro’s law did not always hold true in some rare instances, Avogadro’s hypothesis finally became the accepted theory at the time.
