- Electrons In Calcium 20
- Electrons In Calcium
- Number Of Protons In Calcium
- Electrons In Calcium 43
- Number Of Electrons In Calcium
Well, firstly, you need to know what calcium carbonate is. I can tell you that calcium carbonate is Ca(calcium)C(carbon)O3(3 oxygen). Then you need to know the weight of CaCO3. The weight of Calcium is 40 amu (atomic mass units), the weight of Car. Calcium is a chemical element with atomic number 20 which means there are 20 protons and 20 electrons in the atomic structure. The chemical symbol for Calcium is Ca. Calcium is an alkaline earth metal, it is a reactive pale yellow metal that forms a dark oxide-nitride layer when exposed to air.


Electrons In Calcium 20
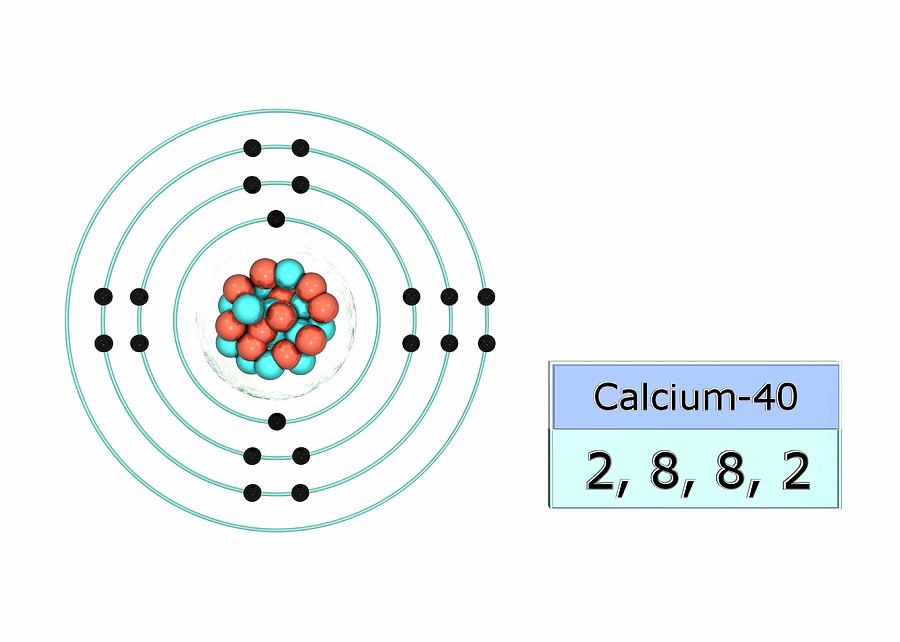
What is the valence electron configuration for calcium?
1 Answer
Calcium can be found in the 4th energy level (row) of the periodic table, and in the 2nd group (column). The first two groups (columns) of the periodic table represent the 's' orbital group. This means that the electron configuration for calcium must end with
The total electron configuration is:
Electrons In Calcium
The valence electrons are found in the highest energy level of the electron configuration in the 's' and 'p' orbitals.
Number Of Protons In Calcium
In the case of calcium this is
Electrons In Calcium 43
I hope this was helpful.
SMARTERTEACHER
Number Of Electrons In Calcium
Related questions
