Indeed, the toxicity level of selenium to humans was established only 20 years ago by studies of Chinese victims of selenium poisoning, selenosis, who grew corn on selenium rich coal rocks. Selenosis has some lovely symptoms: a garlic odor on the breath, hair loss, sloughing of nails, fatigue, irritability, and eventually cirrhosis of the liver.
Element Selenium - Se
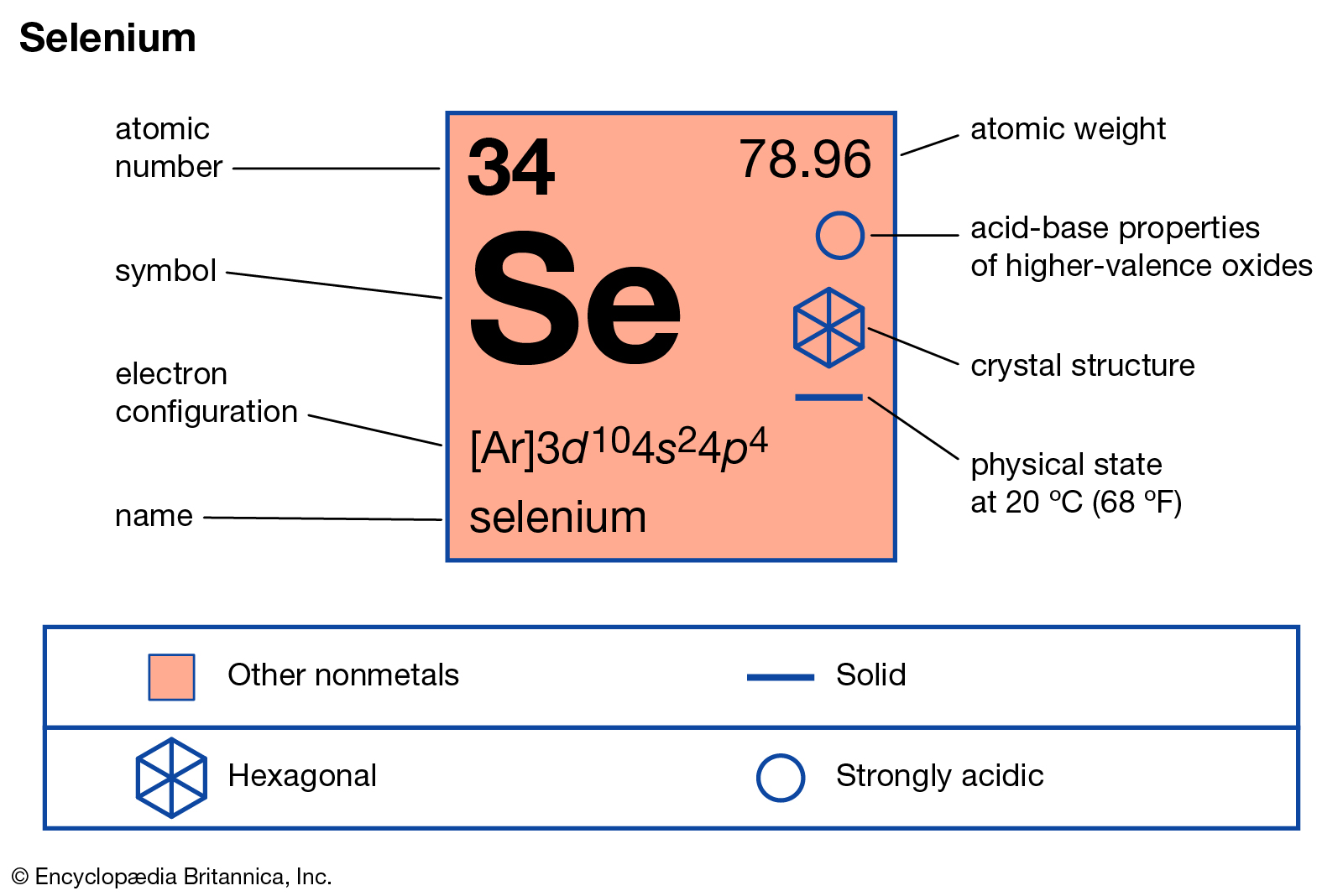
Selenium has six valence electrons. What is the valence of selenium. The number of valence electrons plus the valence equals the. The sum minus the. He valence of selenium depends on which compound it is in. Selenium is very similar to sulfur. It may have a valence of 6; example selenium hexafluoride SeF6, selenium trioxide SeO3 4; example selenium tetrafluoride SeF4, selenium dioxide SeO2 2; example selenium difluoride SeF2, selenium dichloride SeCl2 and - 2; example hydrogen selenide H2Se. Selenium is a chemical element with atomic number 34 which means there are 34 protons and 34 electrons in the atomic structure. The chemical symbol for Selenium is Se. Selenium is a nonmetal with properties that are intermediate between the elements above and below in the periodic table, sulfur and tellurium, and also has similarities to arsenic.
Comprehensive data on the chemical element Selenium is provided on this page; including scores of properties, element names in many languages, most known nuclides of Selenium. Common chemical compounds are also provided for many elements. In addition technical terms are linked to their definitions and the menu contains links to related articles that are a great aid in one's studies.
Selenium Menu
- Selenium Page One
- Selenium Page Two
- Selenium Page Three
Overview of Selenium
- Atomic Number: 34
- Group: 16
- Period: 4
- Series: Nonmetals
Selenium's Name in Other Languages
- Latin: Selenium
- Czech: Selen
- Croatian: Selenij
- French: Sélénium
- German: Selen - r
- Italian: Selenio
- Norwegian: Selen
- Portuguese: Selênio
- Russian: Селен
- Spanish: Selenio
- Swedish: Selen
Atomic Structure of Selenium
- Atomic Radius: 1.22Å
- Atomic Volume: 16.45cm3/mol
- Covalent Radius: 1.16Å
- Cross Section (Thermal Neutron Capture) σa/barns: 11.7
- Crystal Structure: Hexagonal
- Electron Configuration:
- 1s2 2s2p6 3s2p6d10 4s2p4
- Electrons per Energy Level: 2,8,18,6
- Shell Model
- Shell Model
- Ionic Radius: 0.5Å
- Filling Orbital: 4p4
- Number of Electrons (with no charge): 34
- Number of Neutrons (most common/stable nuclide): 45
- Number of Protons: 34
- Oxidation States: ±2,4,6
- Valence Electrons: 4s2p4
- Electron Dot Model
- Electron Dot Model
Chemical Properties of Selenium
- Electrochemical Equivalent: 0.7365g/amp-hr
- Electron Work Function: 5.9eV
- Electronegativity: 2.55 (Pauling); 2.48 (Allrod Rochow)
- Heat of Fusion: 6.694kJ/mol
- Incompatibilities:
- Acids, strong oxidizers, chromium trioxide, potassium bromate, cadmium
- Ionization Potential
- First: 9.752
- Second: 21.19
- Third: 30.82
- Valence Electron Potential (-eV): 120
Physical Properties of Selenium
- Atomic Mass Average: 78.96
- Boiling Point: 958K 685°C 1265°F
- Coefficient of lineal thermal expansion/K-1: 36.9E-6
- Conductivity
- Electrical: 1.0E-12 106/cm Ω
Thermal: 0.0204 W/cmk
- Electrical: 1.0E-12 106/cm Ω
- Density: 4.79g/cc @ 300K
- Description:
- Dark gray lustrous rods or dark red crystals of non-metal. Burns in contact with air but is unaffected by water. Disolves in alkalis and concentrated HNO3.
- Elastic Modulus:
- Bulk: 8.3/GPa
- Rigidity: 3.7/GPa
- Youngs: 58/GPa
- Enthalpy of Atomization: 205.9 kJ/mole @ 25°C
- Enthalpy of Fusion: 6.69 kJ/mole
- Enthalpy of Vaporization: 26.3 kJ/mole
- Flammablity Class: Combustible Solid
- Freezing Point:see melting point
- Hardness Scale
- Brinell: 736 MN m-2
- Mohs: 2
- Heat of Vaporization: 37.7kJ/mol
- Melting Point: 494K 221°C 430°F
- Molar Volume: 16.42 cm3/mole
- Optical Refractive Index: 1.000895
- Physical State (at 20°C & 1atm): Solid
- Specific Heat: 0.32J/gK
- Vapor Pressure = 0.695Pa@221°C
Regulatory / Health
- CAS Number
- 7782-49-2 powder
- UN/NA ID and ERG Guide Number
- 2658 / 152 powder
- RTECS: VS7700000
- OSHAPermissible Exposure Limit (PEL)
- TWA: 0.2 mg/m3
- OSHA PEL Vacated 1989
- TWA: 0.2 mg/m3
- NIOSHRecommended Exposure Limit (REL)
- TWA: 0.2 mg/m3
- IDLH: 1 mg/m3
- Routes of Exposure: Inhalation; Ingestion; Skin and/or eye contact
- Target Organs: Eyes, skin, respiratory system, liver, kidneys, blood, spleen
- Levels In Humans:
Note: this data represents naturally occuring levels of elements in the typical human, it DOES NOT represent recommended daily allowances.- Blood/mg dm-3: 0.171
- Bone/p.p.m: 1-9
- Liver/p.p.m: 0.35-2.4
- Muscle/p.p.m: 0.42-1.9
- Daily Dietary Intake: 0.006-0.2 mg
- Total Mass In Avg. 70kg human: 10-65 mg
Who / Where / When / How
- Discoverer: Jöns J. Berzelius
- Discovery Location: Stockholm Sweden
- Discovery Year: 1817
- Name Origin:
- Greek: Selênê (Moon)
- Abundance of Selenium:
- Earth's Crust/p.p.m.: 0.05
- Seawater/p.p.m.:
- Atlantic Suface: 4.6E-08
- Atlantic Deep: 1.8E-07
- Pacific Surface: 1.5E-08
- Pacific Deep: 1.65E-07
- Atmosphere/p.p.m.: N/A
- Sun (Relative to H=1E12): N/A
- Sources of Selenium:
- Obtained as a by-product of lead, copper and nickel refining. World wide annual production is around 600 tons. Primary mining areas are Canada, USA, Bolivia and Russia.
- Uses of Selenium:
- Used in photoelectric cells, TV cameras, as a semiconductor in solar batteries, light meters, copy machines, anti-dandruff shampoo and rectifiers. Also colors glass red.
- Additional Notes:
- A dose of selenium as small as 5 mg per day can be lethal for many humans.
Selenium Menu
- Selenium Page One
- Selenium Page Two
- Selenium Page Three
References

A list of reference sources used to compile the data provided on our periodic table of elements can be found on the main periodic table page.
Related Resources
- Anatomy of the Atom
Answers many questions regarding the structure of atoms. - Molarity, Molality and Normality
Introduces stoichiometry and explains the differences between molarity, molality and normality. - Molar Mass Calculations and Javascript Calculator
Molar mass calculations are explained and there is a JavaScript calculator to aid calculations. - Chemical Database
This database focuses on the most common chemical compounds used in the home and industry.
Citing this page
If you need to cite this page, you can copy this text:
Kenneth Barbalace. Periodic Table of Elements - Selenium - Se. EnvironmentalChemistry.com. 1995 - 2021. Accessed on-line: 4/24/2021
https://EnvironmentalChemistry.com/yogi/periodic/Se.html
.
Selenium Number Valence Electrons
Linking to this page
If you would like to link to this page from your website, blog, etc., copy and paste this link code (in red) and modify it to suit your needs:
<a href='https://EnvironmentalChemistry.com/yogi/periodic/Se.html'>echo Periodic Table of Elements: Selenium - Se (EnvironmentalChemistry.com)</a>- Comprehensive information for the element Selenium - Se is provided by this page including scores of properties, element names in many languages, most known nuclides and technical terms are linked to their definitions.
.
NOTICE: While linking to articles is encouraged, OUR ARTICLES MAY NOT BE COPIED TO OR REPUBLISHED ON ANOTHER WEBSITE UNDER ANY CIRCUMSTANCES.
PLEASE, if you like an article we published simply link to it on our website do not republish it.

3.1 Two Types of Bonding
Learning Objectives
- Define the octet rule.
- Describe how ionic bonds are formed.
Atoms can join together by forming a chemical bondA very strong attraction between two atoms., which is a very strong attraction between two atoms. Chemical bonds are formed when electrons in different atoms interact with each other to make an arrangement that is more stable than when the atoms are apart.
What causes atoms to make a chemical bond with other atoms, rather than remaining as individual atoms? A clue comes by considering the noble gas elements, the rightmost column of the periodic table. These elements—helium, neon, argon, krypton, xenon, and radon—do not form compounds very easily, which suggests that they are especially stable as lone atoms. What else do the noble gas elements have in common? Except for helium, they all have eight valence electrons. Chemists have concluded that atoms are especially stable if they have eight electrons in their outermost shell. This useful rule of thumb is called the octet ruleThe idea that atoms tend to have eight electrons in their valence shell., and it is a key to understanding why compounds form.
Note
Of the noble gases, only krypton, xenon, and radon have been found to make compounds.
There are two ways for an atom that does not have an octet of valence electrons to obtain an octet in its outer shell. One way is the transfer of electrons between two atoms until all atoms have octets. Because some atoms will lose electrons and some atoms will gain electrons, there is no overall change in the number of electrons, but individual atoms acquire a nonzero electric charge. Those that lose electrons become positively charged, and those that gain electrons become negatively charged. Charged atoms are called ionsA charged atom.. Because opposite charges attract (while like charges repel), these oppositely charged ions attract each other, forming ionic bondsAn attraction between oppositely charged ions.. The resulting compounds are called ionic compoundsA compound formed with an ionic bond. and are the primary subject of this chapter.
The second way for an atom to obtain an octet of electrons is by sharing electrons with another atom. These shared electrons simultaneously occupy the outermost shell of more than one atom. The bond made by electron sharing is called a covalent bond. Covalent bonding and covalent compounds will be discussed in Chapter 4 'Covalent Bonding and Simple Molecular Compounds'.
Note
Despite our focus on the octet rule, we must remember that for small atoms, such as hydrogen, helium, and lithium, the first shell is, or becomes, the outermost shell and hold only two electrons. Therefore, these atoms satisfy a “duet rule” rather than the octet rule.
Example 1
A sodium atom has one valence electron. Do you think it is more likely for a sodium atom to lose one electron or gain seven electrons to obtain an octet?
Solution
Although either event is possible, a sodium atom is more likely to lose its single valence electron. When that happens, it becomes an ion with a net positive charge. This can be illustrated as follows:
| Sodium atom | Sodium ion | ||
|---|---|---|---|
| 11 protons | 11+ | 11 protons | 11+ |
| 11 electrons | 11− | 10 electrons | 10− |
| 0 overall charge | +1 overall charge | ||
Skill-Building Exercise
A fluorine atom has seven valence electrons. Do you think it is more likely for a fluorine atom to lose seven electrons or gain one electron to obtain an octet?
Concept Review Exercises
How are ionic bonds formed?
Selenium Valence Electron Shell
Answers
Selenium Valence Electrons Group
The octet rule is the concept that atoms tend to have eight electrons in their valence electron shell.
Ionic bonds are formed by the attraction between oppositely charged ions.
Selenide Valence Electrons
Key Takeaways
- Atoms have a tendency to have eight electrons in their valence shell.
- The attraction of oppositely charged ions is what makes ionic bonds.
Exercises
Why is an ionic compound unlikely to consist of two positively charged ions?
Why is an ionic compound unlikely to consist of two negatively charged ions?
A calcium atom has two valence electrons. Do you think it will lose two electrons or gain six electrons to obtain an octet in its outermost electron shell?
An aluminum atom has three valence electrons. Do you think it will lose three electrons or gain five electrons to obtain an octet in its outermost electron shell?
A selenium atom has six valence electrons. Do you think it will lose six electrons or gain two electrons to obtain an octet in its outermost electron shell?
An iodine atom has seven valence electrons. Do you think it will lose seven electrons or gain one electron to obtain an octet in its outermost electron shell?
Selenium Valence Shell
Answers
Selenium Dichloride Valence Electrons
Positive charges repel each other, so an ionic compound is not likely between two positively charged ions.
It is more likely to lose two electrons.
It is more likely to gain two electrons.
